Delve into the fascinating world of chemistry with our comprehensive Magnesium Oxide Lab Answer Key, a treasure trove of knowledge that unravels the secrets of this remarkable compound. Embark on a scientific adventure as we explore the intricacies of magnesium oxide’s formation, applications, and more, all presented in an engaging and accessible manner.
Prepare to witness the magic of chemical reactions unfold as we delve into the depths of magnesium oxide’s synthesis, unraveling the mysteries of heat’s role and the products that emerge from this captivating process. Dive into a realm of calculations and analysis, where precision reigns supreme, as we guide you through the intricacies of determining theoretical yield and unravel the significance of accurate measurements.
Magnesium Oxide Lab Overview: Magnesium Oxide Lab Answer Key
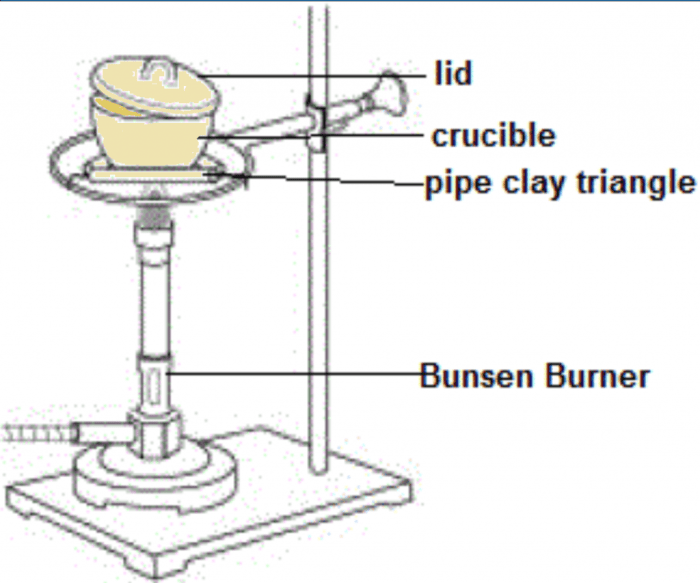
The magnesium oxide lab experiment aims to synthesize magnesium oxide (MgO) through a chemical reaction between magnesium and oxygen. This experiment provides a practical demonstration of the principles of combustion, oxidation-reduction reactions, and the formation of ionic compounds.
Materials and Equipment
The materials and equipment used in the lab include:
- Magnesium ribbon
- Bunsen burner
- Crucible and crucible lid
- Electronic balance
- Safety goggles
- Tongs
Experimental Procedure
The experimental procedure involves the following steps:
- Weigh a clean and dry crucible and lid.
- Cut a piece of magnesium ribbon and weigh it.
- Place the magnesium ribbon in the crucible and cover it with the lid.
- Heat the crucible with a Bunsen burner until the magnesium ignites and burns completely.
- Allow the crucible to cool and weigh it again with the MgO product.
Chemical Reactions Involved
The formation of magnesium oxide involves a chemical reaction between magnesium and oxygen. This reaction is a combination reaction, where two or more substances combine to form a single product.
The balanced chemical equation for the formation of magnesium oxide is:
Mg + O2→ 2MgO
While looking for the magnesium oxide lab answer key, you may have also come across something called what is splendid greens cava . That’s a whole other topic, but if you’re curious, it’s a type of sparkling water that’s said to have some health benefits.
Anyway, back to the magnesium oxide lab answer key…
In this reaction, magnesium (Mg) reacts with oxygen (O 2) to form magnesium oxide (MgO). The reaction is exothermic, meaning that heat is released during the reaction.
Role of Heat in the Reaction
Heat plays a crucial role in the formation of magnesium oxide. The reaction between magnesium and oxygen is a highly exothermic reaction, releasing a significant amount of heat. This heat helps to drive the reaction forward and overcome the activation energy required for the reaction to occur.
Without the presence of heat, the reaction between magnesium and oxygen would be much slower and less efficient. The heat released during the reaction helps to break the bonds between the magnesium and oxygen atoms, allowing them to combine and form magnesium oxide.
Products Formed in the Reaction
The product formed in the reaction between magnesium and oxygen is magnesium oxide (MgO). Magnesium oxide is a white, crystalline solid that is insoluble in water. It is a basic oxide and reacts with acids to form salts.
Magnesium oxide is used in a variety of applications, including as a refractory material, a fertilizer, and an antacid. It is also used in the production of glass, ceramics, and other materials.
Calculations and Analysis
Determining the theoretical yield of magnesium oxide is crucial for evaluating the efficiency of the synthesis reaction.
Calculating Theoretical Yield
The theoretical yield is the maximum amount of product that can be obtained from a given amount of reactants, assuming a complete reaction and no losses.
- Step 1:Determine the balanced chemical equation for the reaction. For the magnesium oxide synthesis, the equation is: 2Mg + O 2→ 2MgO.
- Step 2:Calculate the molar mass of magnesium oxide (MgO). This is 40.30 g/mol.
- Step 3:Convert the given mass of magnesium to moles. For example, if you have 5.0 g of magnesium, the moles of magnesium would be 5.0 g / 24.31 g/mol = 0.206 mol.
- Step 4:Use the stoichiometry of the balanced equation to determine the moles of magnesium oxide produced. From the equation, 2 moles of magnesium produce 2 moles of magnesium oxide. Therefore, 0.206 mol of magnesium will produce 0.206 mol of magnesium oxide.
- Step 5:Convert the moles of magnesium oxide to grams. Multiply the moles by the molar mass of magnesium oxide: 0.206 mol – 40.30 g/mol = 8.32 g.
Therefore, the theoretical yield of magnesium oxide for 5.0 g of magnesium is 8.32 g.
Importance of Accuracy
Accuracy in the calculations is essential because it ensures a reliable prediction of the maximum amount of product that can be obtained. Errors in calculations can lead to incorrect conclusions about the efficiency of the reaction and potential losses during the synthesis.
Data Interpretation and Discussion

Interpreting the experimental data involves analyzing the mass changes and calculating the percentage composition of magnesium oxide (MgO) in the sample. The mass of the crucible and lid before and after heating provides insights into the changes that occurred during the experiment.
Sources of Error
Several factors can introduce errors into the experiment:
- Incomplete combustion: Failure to completely burn the magnesium ribbon can lead to underestimation of the mass of MgO formed.
- Absorption of moisture: The crucible and lid may absorb moisture from the air, resulting in an overestimation of the mass of MgO.
- Inaccurate weighing: Errors in weighing the crucible and lid can affect the accuracy of the mass change calculations.
Improving Accuracy
To improve the accuracy of the results, several measures can be taken:
- Ensure complete combustion by burning the magnesium ribbon until it glows brightly and no further mass change is observed.
- Store the crucible and lid in a desiccator before weighing to minimize moisture absorption.
- Use a high-precision balance to ensure accurate mass measurements.
Applications of Magnesium Oxide
Magnesium oxide is a versatile material with a wide range of applications across various industries. Its unique properties, such as high thermal conductivity, electrical insulation, and chemical inertness, make it suitable for use in a variety of products and processes.
Refractory Materials
Magnesium oxide’s high melting point and thermal stability make it an ideal material for use in refractory applications, such as linings for furnaces and kilns. It can withstand extreme temperatures and harsh chemical environments, ensuring the durability and efficiency of these industrial processes.
Construction Materials
Magnesium oxide is commonly used in the construction industry as a fire retardant and insulating material. It is incorporated into building materials, such as wallboards, ceiling tiles, and insulation, to improve fire resistance and reduce heat transfer.
Agriculture, Magnesium oxide lab answer key
In agriculture, magnesium oxide is used as a soil amendment to supplement magnesium levels in the soil. Magnesium is an essential nutrient for plant growth, and magnesium oxide provides a slow-release source of this nutrient, helping to improve crop yields and plant health.
Water Treatment
Magnesium oxide is used in water treatment processes to remove impurities and improve water quality. It can act as a coagulant, helping to form flocs that trap contaminants and allow them to be easily removed from the water.
Medical Applications
In the medical field, magnesium oxide is used as an antacid to neutralize stomach acid and relieve heartburn. It is also used as a laxative and in some cases as a magnesium supplement.
Top FAQs
What is the purpose of the magnesium oxide lab experiment?
The magnesium oxide lab experiment aims to demonstrate the formation of magnesium oxide through a chemical reaction and to provide hands-on experience in performing chemical calculations.
What materials are required for the magnesium oxide lab?
The materials typically required for the magnesium oxide lab include magnesium ribbon, oxygen gas, a crucible, a Bunsen burner, and a balance.
How is magnesium oxide formed in the lab?
Magnesium oxide is formed in the lab through a combustion reaction between magnesium and oxygen. The magnesium ribbon is heated in the presence of oxygen gas, resulting in the formation of magnesium oxide.