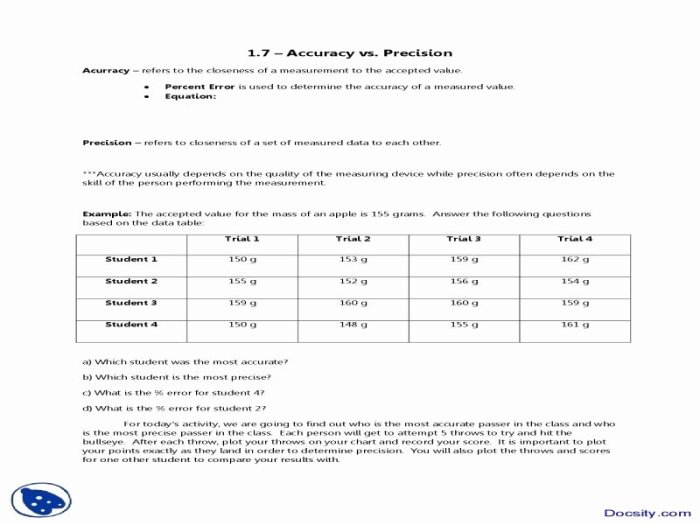
Introducing the Accuracy and Precision Chemistry Worksheet, an invaluable resource designed to enhance your understanding of measurement reliability. This comprehensive guide delves into the fundamental concepts of accuracy and precision, empowering you with the knowledge and skills to make informed decisions and ensure the integrity of your experimental results.
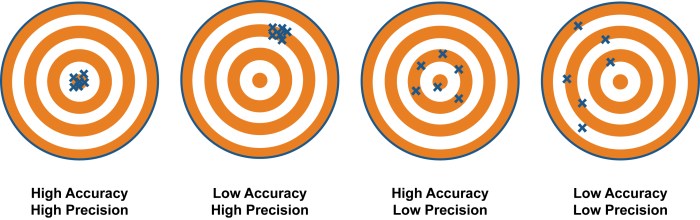
Accuracy, the closeness of a measurement to its true value, and precision, the consistency of repeated measurements, are essential pillars of scientific inquiry. By mastering these concepts, you will gain a deeper appreciation for the nuances of measurement and its impact on the reliability of your data.
Accuracy and Precision in Chemistry
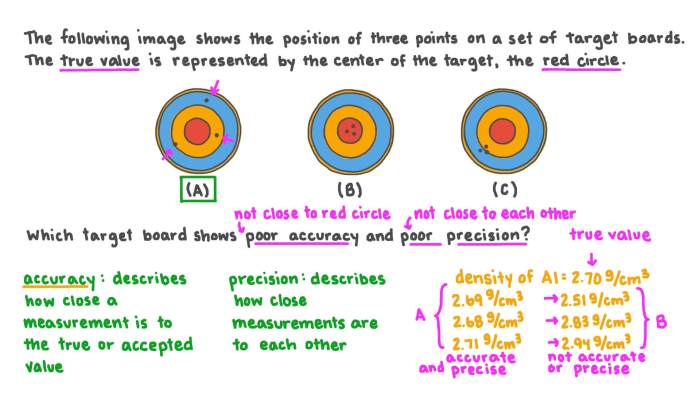
Accuracy and precision are two important concepts in chemistry that are often used to describe the quality of a measurement. Accuracy refers to how close a measurement is to the true value, while precision refers to how consistent a set of measurements is.
Factors Affecting Accuracy and Precision
- Instrument calibration:Uncalibrated or poorly calibrated instruments can lead to inaccurate measurements.
- Environmental conditions:Temperature, humidity, and other environmental factors can affect the accuracy and precision of measurements.
- Operator technique:The skill and experience of the operator can influence the accuracy and precision of measurements.
Methods to Improve Accuracy and Precision, Accuracy and precision chemistry worksheet
- Use calibrated instruments:Regularly calibrate instruments to ensure they are providing accurate measurements.
- Control environmental conditions:Maintain a stable temperature and humidity in the measurement environment.
- Train operators:Provide proper training to operators to ensure they are using instruments correctly and consistently.
Applications of Accuracy and Precision in Chemistry
Accuracy and precision are essential in chemical analysis because they impact the reliability of experimental results. Accurate and precise measurements are crucial in:
- Quantitative analysis:Determining the concentration of a substance in a sample.
- Spectroscopic analysis:Identifying and characterizing compounds based on their absorption or emission spectra.
- Titrations:Determining the concentration of a solution by adding a known amount of a reagent.
Helpful Answers: Accuracy And Precision Chemistry Worksheet
What is the difference between accuracy and precision?
Accuracy refers to how close a measurement is to its true value, while precision describes the consistency of repeated measurements.
How can I improve the accuracy of my measurements?
To improve accuracy, use calibrated instruments, follow standardized procedures, and minimize sources of error.
What factors can affect the precision of my measurements?
Factors such as instrument limitations, environmental conditions, and operator technique can impact precision.