Ir spectra of 1 methylcyclohexene – IR spectra of 1-methylcyclohexene, a versatile tool in organic chemistry, unveil the intricate molecular structure and functional group composition of this cyclic alkene. This comprehensive guide delves into the characteristic IR bands, their correlation with functional groups, and the applications of IR spectroscopy in analyzing 1-methylcyclohexene.
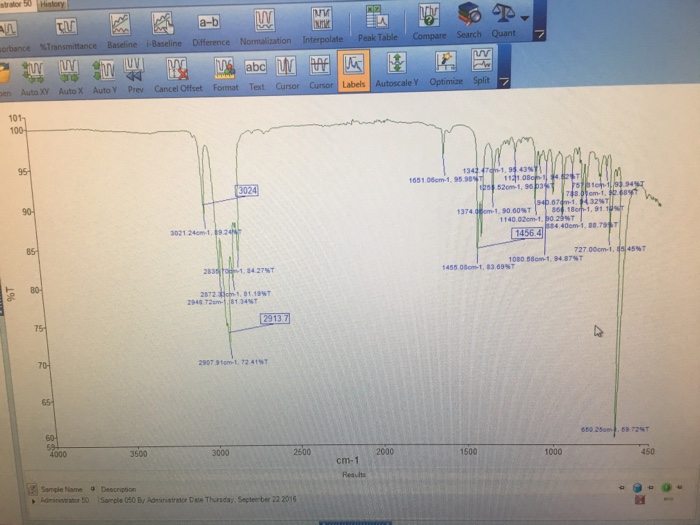
The IR spectrum of 1-methylcyclohexene exhibits distinct absorption bands that provide valuable insights into its molecular structure. The C=C stretching vibration manifests as a prominent band in the 1640-1680 cm-1 region, while the C-H stretching vibrations of the sp3 hybridized carbons appear in the 2850-3000 cm-1 range.
Additionally, the presence of the methyl group is indicated by the C-H stretching vibrations around 2960 cm-1 and the C-H bending vibrations near 1450 cm-1.
IR Spectra of 1-Methylcyclohexene
IR spectroscopy is a powerful technique used to identify and characterize organic compounds. It involves the absorption of infrared radiation by a molecule, which causes the excitation of specific vibrational modes. The IR spectrum of a compound is a plot of the absorbance or transmittance of infrared radiation as a function of wavelength or wavenumber.
Alkenes, which contain a carbon-carbon double bond, exhibit characteristic IR bands that can be used to identify their presence in a molecule. These bands arise from the stretching vibrations of the C=C double bond and the C-H bonds adjacent to the double bond.
IR Spectrum of 1-Methylcyclohexene
1-Methylcyclohexene is a cyclic alkene that exhibits several characteristic IR bands in its IR spectrum:
- C=C Stretching Vibration:A strong band at around 1640 cm -1corresponds to the stretching vibration of the C=C double bond.
- C-H Stretching Vibrations:Bands at around 3075 cm -1and 3000 cm -1correspond to the stretching vibrations of the C-H bonds adjacent to the double bond.
- C-H Bending Vibrations:A band at around 1450 cm -1corresponds to the bending vibrations of the C-H bonds in the methyl group.
- C-C Stretching Vibrations:Bands at around 1260 cm -1and 1160 cm -1correspond to the stretching vibrations of the C-C bonds in the cyclohexane ring.
Comparison with Other Alkenes, Ir spectra of 1 methylcyclohexene
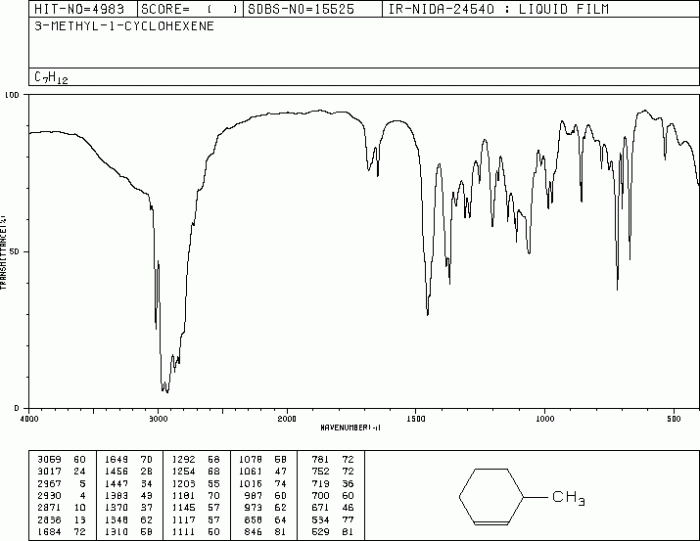
The IR spectrum of 1-methylcyclohexene is similar to that of other alkenes, such as ethene and propene. All alkenes exhibit a strong C=C stretching band in the region of 1640-1680 cm -1.
However, there are some subtle differences between the IR spectra of different alkenes. For example, the C-H stretching bands of 1-methylcyclohexene are shifted to lower wavenumbers compared to those of ethene and propene due to the presence of the cyclohexane ring.
Applications of IR Spectroscopy in Analyzing 1-Methylcyclohexene
IR spectroscopy is a valuable tool for identifying and characterizing 1-methylcyclohexene in various samples. It can be used to:
- Identify the presence of 1-methylcyclohexene in a mixture of organic compounds.
- Determine the purity of 1-methylcyclohexene samples.
- Monitor the reaction progress of reactions involving 1-methylcyclohexene.
- Analyze the composition of complex organic mixtures, such as those found in petroleum or environmental samples.
FAQ Guide: Ir Spectra Of 1 Methylcyclohexene
What is the significance of the C=C stretching vibration in the IR spectrum of 1-methylcyclohexene?
The C=C stretching vibration is a characteristic band that provides direct evidence for the presence of a double bond in the molecule. Its position and intensity can provide insights into the nature of the double bond and its substitution pattern.
How can IR spectroscopy be used to differentiate between 1-methylcyclohexene and other alkenes?
The IR spectra of different alkenes exhibit subtle differences in the position and intensity of their characteristic bands. By comparing the IR spectra of 1-methylcyclohexene with those of other alkenes, such as ethene or propene, it is possible to identify and distinguish between these compounds.
What are some practical applications of IR spectroscopy in analyzing 1-methylcyclohexene?
IR spectroscopy is widely used in various fields to analyze 1-methylcyclohexene. In organic chemistry, it helps identify and characterize this compound in reaction mixtures or complex samples. In environmental analysis, IR spectroscopy can be used to detect 1-methylcyclohexene in air or water samples, providing valuable information about potential pollution sources.